

.png?sfvrsn=1b6bae1a_0) (FASTER)
(FASTER).png?sfvrsn=ac211bda_0)
The global energy demand is increasing day by day. Over 80% of human energy needs come from burning fossil fuels. Among the green-house gases, CO2 from burning the fossil fuels is the main culprit contributing to the global warming. There are a lot of news of erratic weather patterns lately which are linked to global warming. It looks imperative to achieve carbon neutrality in the energy sectors. Around the world, policy-makers and researchers are now seriously considering a shift from conventional fuels to renewable resources for economic, environment and energy security reasons. It is a grand challenge to meet the “dual revolution” in engine technologies and in fuel stream. The new designs of gas turbines and engines are evolving in a rapid pace to achieve high efficiency and low emission for future sustainability. The new designs rely on a number of physical models, of which chemical kinetics plays a critical role. To this end, our group heavily focuses on building an extensive database to characterize the combustion properties of various carbon-neutral and/or low carbon fuels. The database includes, but not limited to ignition delay times, species time-history profiles and reaction rate parameters over a wide range of pressures and temperatures.
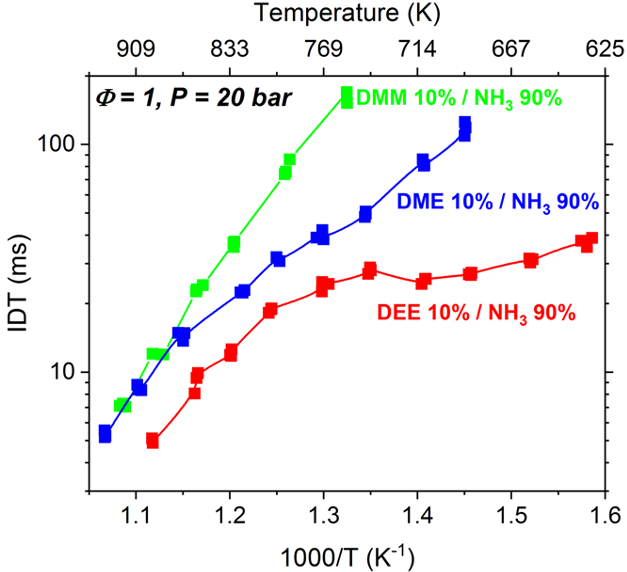
Ours was among the few groups who initiated studying ammonia (NH3) as a zero-carbon fuel (Shu et al., PROCI 2019). Since ammonia is a low-reactivity fuel, we focused on studying blends of ammonia that can enhance the ignition of ammonia
and make it more suitable for practical devices. In particular, we studied blends of ammonia with a family of e-fuels / biofuels, known as di-alkyl-ethers. We found that the blend of ammonia with diethyl ether (DEE) provides superior combustion properties
(Issayve et al., PROCI 2021) as compared to its blends with dimethyl ether (Issayve et al., Renewable Energy 2021) and dimethoxy methane (Elbaz et al., Energy & Fuels 2020). Since one major issue with ammonia is the
high formation of NOx due to the fuel-bound nitrogen, we showed that this issue can be tackled by either carrying out ammonia combustion at fuel-rich conditions or by blending ammonia with a high-reactivity fuel to enable ignition at lower temperatures.
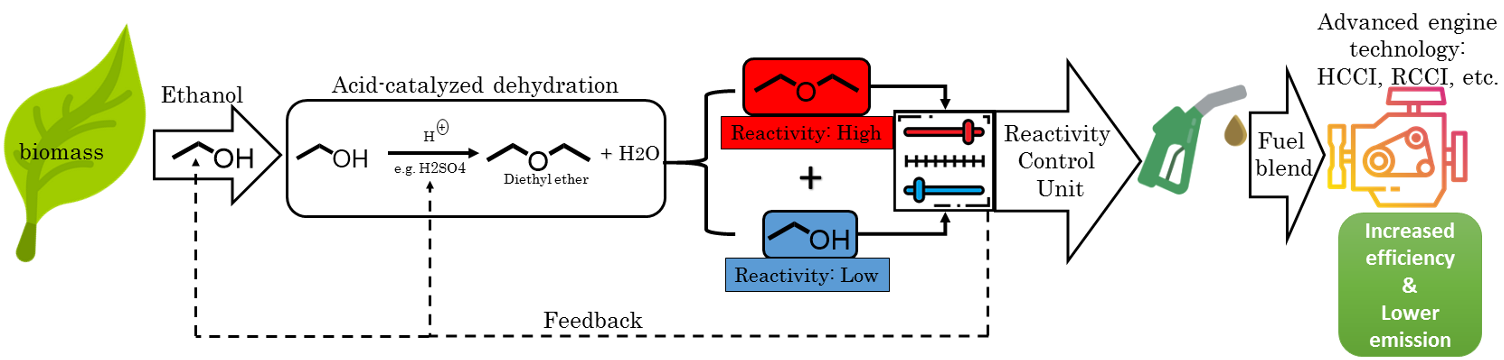
We analyzed blends of DEE and ethanol as these two fuels can be synthetically produced through the same process and their highly divergent ignition properties can be exploited to produce a tailor-made fuel with the desired properties (Issayev et al., FUEL 2020). Recently, we extended this work to explore ignition properties of dibutyl ether which has higher volumetric energy density compared to its small-chain counterparts. This fuel exhibited a highly unique 4-stage ignition at low temperatures (Hakimov et al., Combustion & Flame 2021).
Below are some of our recent publications on zero-carbon fuels, biofuels and e-fuels: