

.png?sfvrsn=1b6bae1a_0) (FASTER)
(FASTER).png?sfvrsn=ac211bda_0)

Schematic structure of a sooting flame and its general formation procedure.
Rapid population growth and industrialization assure rising worldwide energy demands in the coming decades. Although alternative sources, such as wind, solar, geothermal, and nuclear energies, are increasingly important in the world energy mix, conventional petroleum-based fuels, such as natural gas, gasoline, kerosene, and diesel, are still going to play a dominant role in the foreseeable future.
Soot is carbonaceous particle resulting from incomplete combustion of hydrocarbon fuels, which directly impact the combustion inefficiency in practical combustion devices. Being very fine particles with diameters typically less than a micrometer, soot is known to be an important contributor to PM2.5. After being inhaled, ultrafine soot particles with diameters less than 100 nm (typical flame generated soot) can travel deeply into the lungs, deposit in the alveoli, enter the circulatory system, and finally reach other organs like liver, heart, and brain, causing potential health issues. Soot emission into the atmosphere also has dire consequences to the environment. Soot is a contributor to global warming, second only to CO2. Soot deposition promotes the melting of ice glaciers, soot in the upper atmosphere affects weather in the form of clouds and precipitation by providing condensation nuclei.
The need to suppress soot emission necessitates active control of the soot formation processes, which in turn requires a fundamental understanding of the physicochemical pathways from fuel to soot. Soot formation is one of the most complex phenomena in combustion, involving complicated interactions between combustion chemistry, fluid mechanics, mass/heat transport, and particle dynamics. Sufficient experimental evidence has linked polycyclic aromatic hydrocarbons (PAH) to the soot inception in combustion processes. A number of mechanisms have been proposed to explain the formation and growth of PAHs starting from small hydrocarbons, such as Hydrogen-abstraction acetylene-addition (HACA), hydrogen-abstraction vinylacetylene-addition (HAVA), phenyl addition-dehydrocyclization (PAC) and clustering of hydrocarbons by radical-chain reactions (CHRCR). However, the fundamental formation mechanisms of carbonaceous nanostructures from PAH building blocks remain elusive.
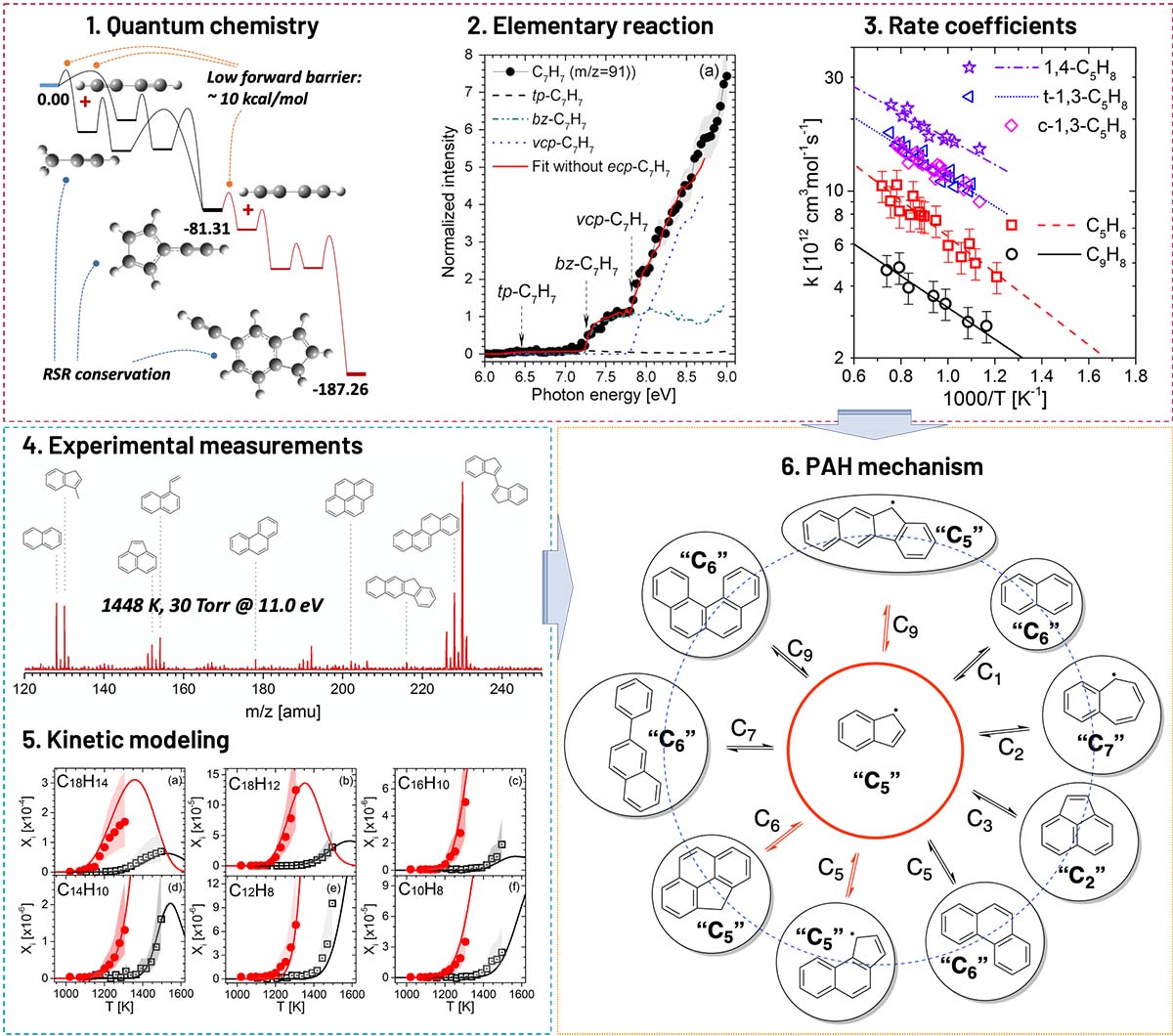
Complete set of methods for the investigation of PAH combustion chemistry.
We have developed a complete set of methods in our group to investigate the combustion chemistry of aromatic hydrocarbons, and to facilitate a comprehensive understanding of PAH and soot formation. As shown in Fig. 2, reaction kinetics are explored with a combination of quantum chemical calculation, elementary reaction validation, and rate coefficient measurements. PAH formation pathways driven by resonance stabilized radicals (RSRs) were studied in the reacting system of vinylacetylene / butadiyne and propargyl (Refs. 1 and 4). We found two C7 RSRs, vinyl-cyclopentadienyl and fulvenallenyl, are the critical intermediates in these RSR driven PAH pathways. In addition, butadiyne is able to unergo continuous addition to RSR without frequent H-elimination, which leads to a quick PAH growth in radical deficient reacting environment. Phenyl combination reaction and H-abstraction of indene by OH radical are crucial elementary reactions for aromatic nucleation and oxidation. Laser spectroscopy combined with reflected shock tube was applied in our experimental measurements of their rate coefficients (Refs. 5 and 9).
All these fundamental investigations provide the base for the kinetic model development for PAH chemistry in combustion. Indene and methyl-naphthalene are two typical bicyclic aromatics that can form the smallest PAH RSRs, indenyl and naphthylmethyl. The pyrolysis of indene and methyl-naphthalene were studied in a flow reactor, the reaction processes were quantitatively analyzed by synchrotron vacuum ultra-violet photoionization mass spectrometry (Refs. 2, 3, 11, and 12). As shown in Fig. 2, the comparison of kinetic modeling and experimental results illustrates and validates the PAH mechanism. Three major types of PAH formation pathways are summarized according to our research: 1) addition and cyclization reactions of naphth-1-yl-methyl and naphth-1-yl radicals; 2) recombination of resonance stabilized radicals (indenyl, benzo-fulvenallenyl, phenalenyl, etc.) and the subsequent ring expansion reactions; 3) sequential propargyl addition reactions. We also investigated ethylene co-flow flame using computational fluid dynamic (CFD) simulation and mass spectrometry (Ref. 6). A nascent layer structured PAH mechanism was generalized for the first time, and further investigations are required to enrich its details and expand its application.
Continuous butadiyne addition to propargyl: a radical-efficient pathway for polycyclic aromatic hydrocarbons
H. Jin, L. Xing, J. Yang, Z. Zhou, F. Qi, A. Farooq
J. Phys. Chem. Lett. (2021) in proof.
Experimental and kinetic modeling study of α-methyl-naphthalene pyrolysis: Part I. Formation of monocyclic aromatics and small species
H. Jin, J. Hao, J. Yang, J. Guo, Y. Zhang, C. Cao, A. Farooq
Combust. Flame, (2021)
Experimental and kinetic modeling study of α-methyl-naphthalene pyrolysis: Part II. PAH formation
H. Jin, J. Hao, J. Yang, J. Guo, Y. Zhang, C. Cao, A. Farooq
Combust. Flame (2021)
First aromatic ring formation by the radical-chain reaction of vinylacetylene and propargyl
H. Jin, L. Xing, D. Liu, J. Hao, J. Yang, A. Farooq
Combust. Flame 225, 524-534 (2021)
A high temperature shock tube study of phenyl recombination reaction using laser absorption spectroscopy
H. Jin, B.R. Giri, D. Liu, A. Farooq
Proc. Combust. Inst. 38, 919-927, (2021)
Experimental and numerical study of polycyclic aromatic hydrocarbon formation in ethylene laminar co-flow diffusion flames
H. Jin, J. Guo, T. Li, Z. Zhou, H.G. Im, A. Farooq
Fuel 289, 119931, (2021)
Rapid soot inception via α-alkynyl substitution of polycyclic aromatic hydrocarbons
P. Liu,1 H. Jin,1 B. Chen, J. Yang, Z. Li, A. Bennett, A. Farooq, S. Mani Sarathy, W.L. Roberts
Fuel 295, 120580, (2021)
A mid-infrared diagnostic for benzene using a tunable difference-frequency-generation laser
M.K. Shakfa, M. Mhanna, H. Jin, D. Liu, K. Djebbi, M. Marangoni, A. Farooq
Proc. Combust. Inst. 38, 1787-1796, (2021)
Chemical kinetics of hydroxyl reactions with cyclopentadiene and indene
H. Jin, D. Liu, J. Zou, J. Hao, C. Shao, M. Sarathy, A. Farooq
Combust. Flame 217, 48-56, (2020)
Determination of absolute photoionization cross-sections of some aromatic hydrocarbons
H. Jin, J. Yang, A. Farooq
Rapid Commun. Mass Spectrom. 34, e8899, (2020)
A chemical kinetic modeling study of indene pyrolysis
H. Jin, L. Xing, J. Hao, J. Yang, Y. Zhang, C. Cao, Y. Pan, A. Farooq
Combust. Flame 206, 1-20, (2019)
An experimental study of indene pyrolysis with synchrotron vacuum ultraviolet photoionization mass spectrometry
H. Jin, J. Yang, L. Xing, J. Hao, Y. Zhang, C. Cao, Y. Pan, A. Farooq
Phys. Chem. Chem. Phys. 21, 5510-5520, (2019)