

.png?sfvrsn=1b6bae1a_0) (FASTER)
(FASTER).png?sfvrsn=ac211bda_0)
In catalytic combustion of light hydrocarbons, noble metal-based catalysts like palladium and platinum have excellent catalytic activity, but they are prone to sintering at high temperatures, leading to deactivation. Their high cost and limited availability also hinder widespread use. Conversely, non-noble metal catalysts have favorable thermal stability, excellent oxygen activation, and lower costs, but their catalytic activity is generally lower than that of noble metals. This project aims to enhance noble metal catalyst performance and explore novel combinations of non-noble metals to improve
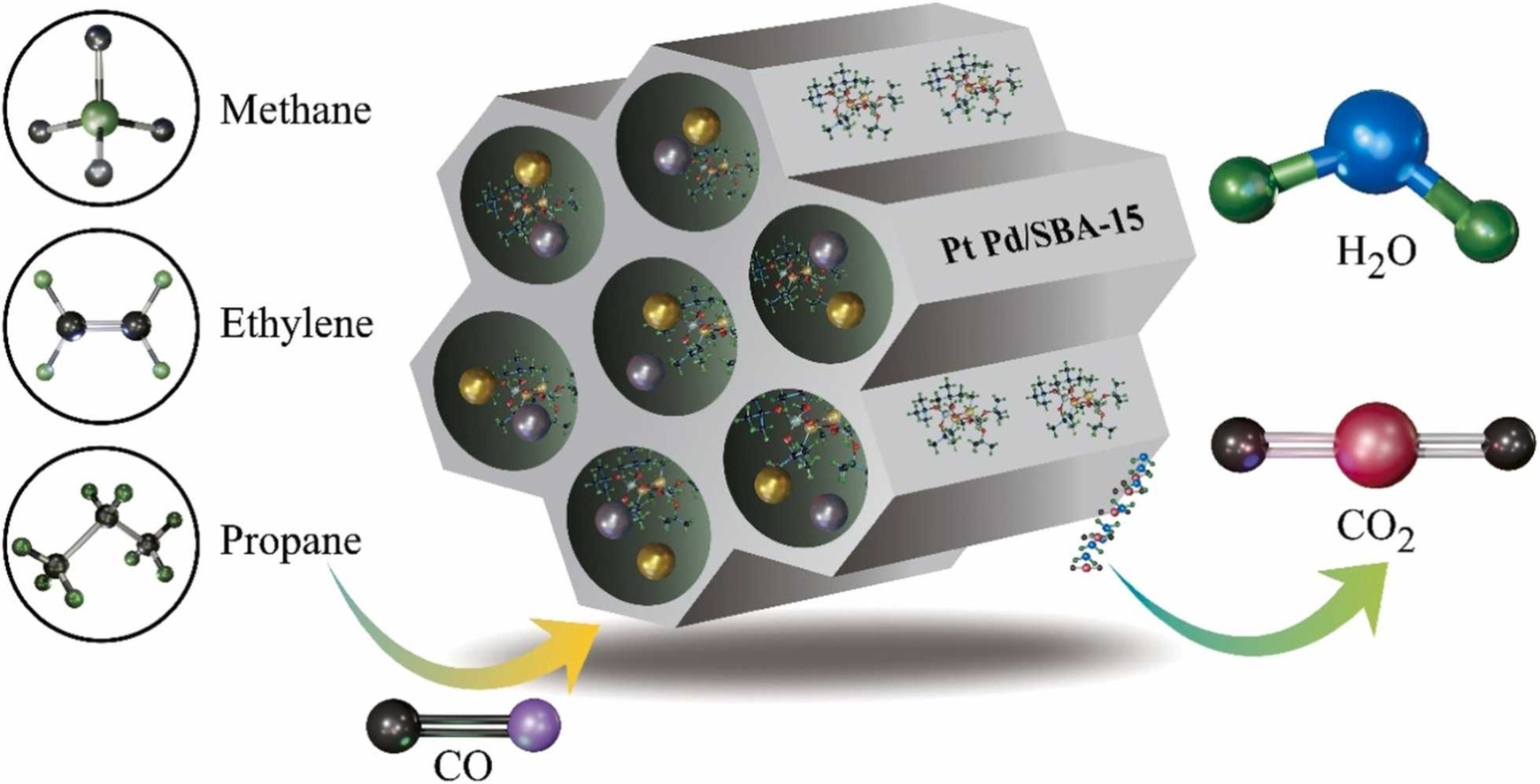
PtPd supported on SBA-15 for wet CO and hydrocarbon oxidation
Light hydrocarbons are promising alternative energy sources that could potentially replace traditional fossil fuels. However, the unburned emission of these light hydrocarbon molecules in industrial applications can cause significant environmental problems due to their high global warming potential. As a result, the removal of unburned light hydrocarbon molecules has received extensive attention. Catalytic combustion technology is widely used for their removal due to its cost-effectiveness, low energy consumption, high removal efficiency, mild reaction conditions, and non-toxic, harmless products.
HAADF and TEM EDX of pristine Pd/Zr0.33Ce0.33/n-Si0.33O2 (a1–a6) and spent Pd/Zr0.33Ce0.33/n-Si0.33O2 (b1–b6) catalyst at 350 °C for 35 hr.
Impact:
The research aims to develop cost-effective and scalable catalytic systems for hydrocarbon emission control. By advancing catalytic combustion technology, the project aspires to contribute to a cleaner and more sustainable industrial landscape, reducing environmental impact and improving economic feasibility of emission control technologies.