

.png?sfvrsn=1b6bae1a_0) (FASTER)
(FASTER).png?sfvrsn=ac211bda_0)
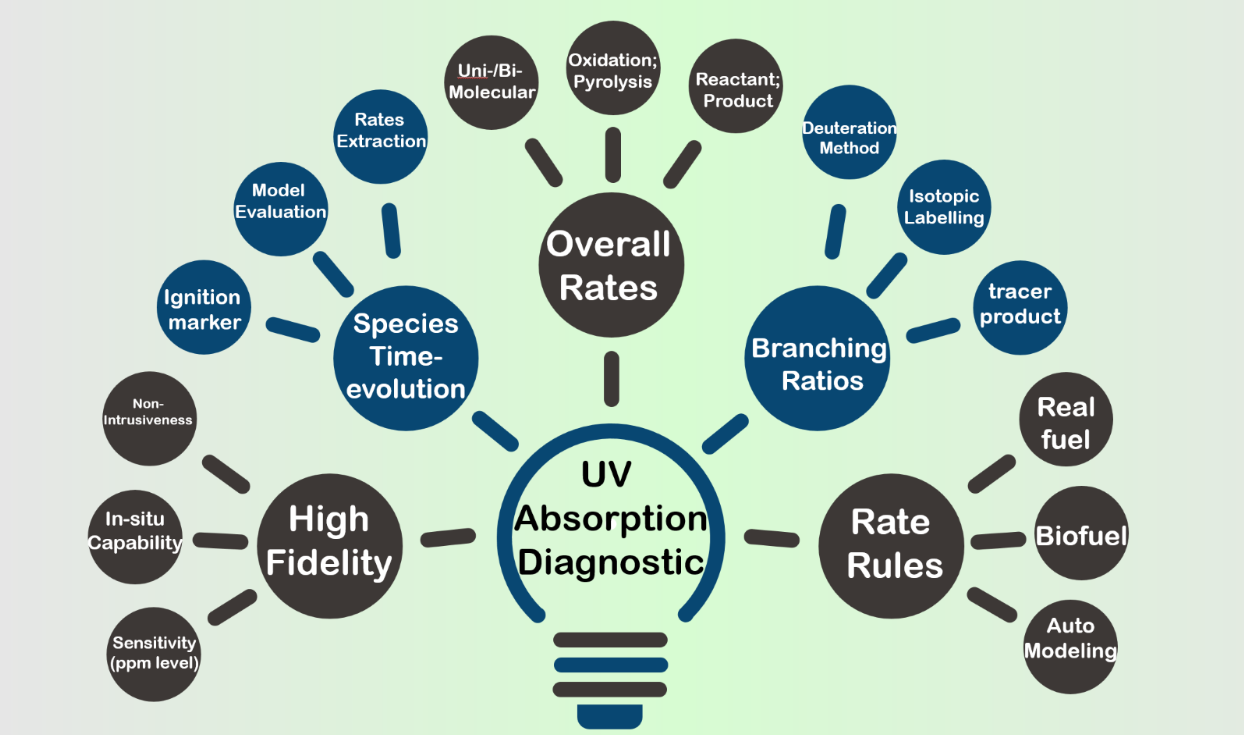
The increasingly stringent emission regulations and a limited energy reservoir asks for sustainable energy source and novel combustion mode. The fidelity of relevant combustion models significantly impacts our evaluation of the properties potential biofuels as well as advanced combustion modes. To resolve the blurriness in current literature models and towards a high-fidelity kinetic modeling of conventional and bio-derived fuels. We have conducted the following works:
Sensitive UV absorption diagnostic exhibit unique advantage of high sensitivity, in addition to the non-intrusiveness and in-situ capability of common advantages of general absorption diagnostics. Furthermore, the sub-microseconds sampling rate allows measurements of fast-evolving radicals and time-evolution of stable species, which is absent in static species measurements technique such as GC and MS. Notably, our diagnostic scheme of allyl radical (at 220 nm) and OH radical (near 306.68 nm) achieves a detection limit of ppm level at high temperatures, from a single-pass configuration (absorption path ~ 14.2 cm) in the shock tube. Impressively, our diagnostic scheme for 1,3-butadiene at 212.5 nm is over 60 times more sensitive than its strongest IR diagnostic scheme. Such sensitive diagnostics enabled our measurements to be conducted in highly diluted test mixtures, which minimized the interference induced by reaction enthalpy and favors constant-temperature test conditions.
Time-histories of important species such as OH not only serves as marker of macro events such as ignition, but also work as solid targets for kinetic model evaluation. Additionally, they provide solid foundation for rate coefficients determination of important reactions. The sensitive UV absorption in the shock tube proves powerful tool in measuring the time-histories of crucial species at accurate test conditions and without the interference of transportation phenomenon.
Overall rate coefficients reported in literature might exhibit significant discrepancies that span by orders of magnitude. High-fidelity measurements of such important reactions are warrantied to resolve the discrepancies in literatures. Employing these sensitive UV absorption diagnostics, we have designed and conducted rate coefficients measurement for important uni- and bi-molecular reactions, which greatly resolved the discrepancies in literature, and improved the model accuracy for both pyrolysis and oxidation conditions, for both conventional and bio-derived fuel.
Branching ratios: The majority of molecules in real fuel contains multiple chemical bonds, and consequently, the important reactions in their combustion could proceed in multiple pathways, leading to distinct products. The channel-specific competition of various reaction pathways is imperative impacting the combustion properties of real fuel. In addition to the contribution to kinetic aspects, we have additionally tested three methods for measuring the branching ratios of reactions., including the “deutration method” and the “tracer species” method.
Rate rules: Direct measurements of crucial reactions for large hydrocarbons presents significant challenges including low vapor pressure and increasing potential interference of secondary reactions. However, large hydrocarbons constitute a significant portion of real fuels which necessities knowledge of their kinetics. The rate rules that’s established from the measurements of smaller molecules which possess similar chemical features might provide reasonably well estimation. Moreover, the rate rules are also necessary for automatic kinetic modeling studies, which is needed considering the numerous amount of species in real fuels and limited researcher resources. We have so far developed three set of rate rules covering the reactions of OH + alkane, OH + alkene, and decomposition reactions of alkene. Implementing our rate rules to existing literature models has resulted in improvement in their prediction accuracy.
Heptanone isomers as biofuel: reactivity with OH radical,
D. Liu & al., Proceedings of the Combustion Institute.
Investigation of Cyclopentene Dehydrogenation: Unimolecular H2 elimination and H abstraction by OH,
D. Liu & al., Combustion and Flame.
Allylic-alkylic C-C bond decomposition in 1-butene and 1-pentene,
C. Zhou & al., Combustion and Flame (2024).
Investigation of thermal decomposition of nitrobenzene: An energetic material,
D. Liu & al., Combustion and Flame.
Gamma-valerolactone (GVL) as a biofuel: Investigation of GVL termal decomposition and GVL+ OH reaction,
D. Liu & al., Combustion and Flame.
Investigation of cyclohexene thermal decomposition and cyclohexene+ OH reactions,
D. Liu & al., Combustion and Flame.
Investigation of the kinetics of conjugated diolefins using UV absorption spectroscopy,
D. Liu & al., Proceedings of the Combustion Institute .
Reaction kinetics of OH radicals with 1, 3, 5-trimethyl benzene: An experimental and theoretical study,
D. Liu & al., Proceedings of the Combustion Institute.
A high temperature shock tube study of phenyl recombination reaction using laser absorption spectroscopy,
H. Jin & al.,Proceedings of the Combustion Institute.
Kinetics and thermochemistry of cyclohexadienes reactions with hydroxyl radicals,
D. Liu & al., Proceedings of the Combustion Institute.
On the redox reactions between allyl radicals and NO,
D. Liu & al., Proceedings of the Combustion Institute.
High temperature branching ratio of acetaldehyde +OH reaction,
D. Liu & al., Proceedings of the Combustion Institute.
Chemical kinetics of hydroxyl reactions with cyclopentadiene and indene,
H. Jin & al., Combustion and Flame, (2020).
A shock tube kinetic study of allyl + allyl and allyl + OH recombination reactions at high temperatures,
F. Khaled & al., Proceedings of the Combustion Institute, (2019).
A shock tube kinetic study on the branching ratio of methanol + OH reaction,
D. Liu & al., Proceedings of the Combustion Institute.
H-Abstraction by OH from Large Branched Alkanes: Overall Rate Measurements and Site-Specific Tertiary Rate Calculations,
D. Liu & al., J. Phys. Chem. A,